

Curcumin, a natural compound found in the rhizome of turmeric, has anti-inflammatory and anti-cancer properties. However, the bioavailability of curcumin is very low, which greatly limits its clinical application. In order to solve this problem, many scientists have developed new formulations with improved bioavailability, but they cannot make curcumin exert its maximum anti-tumor effect.

Recently, scientists from Kyoto University in Japan have developed a curcumin prodrug that can improve bioavailability—synthetic curcumin β-D-glucuronide sodium salt (TBP1901), and found that TBP1901 can significantly shrink the curcumin by converting it into curcumin in vivo. Tumor volume in multiple myeloma model mice. The findings were published in the European Journal of Pharmacology.
In this study, the researchers first demonstrated through mouse experiments that β-glucuronidase (GUSB) is the key enzyme that converts TBP1901 into curcumin in mice.
Subsequently, the curcumin conversion rate of TBP1901 in major organs such as bone marrow, lung, heart, liver and kidney was tested in GUSB-containing mice. Thirty minutes after a single supplementation of TBP1901 at a dose of 30 mg/kg, conversion rates of TBP1901 to curcumin were found to be 0.3 (blood), 16.7 (bone marrow), 3.9 (lung), 8.8 (heart), 10 (liver) , 4.9 (kidney). It can be seen that the conversion rate of curcumin in bone marrow is the highest.
The researchers further verified the above conclusions through experiments in cynomolgus monkeys, by injecting TBP1901 (500mg/kg) intravenously, and collecting blood and bone marrow fluid for testing after 1 hour. It was found that the conversion rates of curcumin in bone marrow and blood were 2.8 and 0.12, respectively.
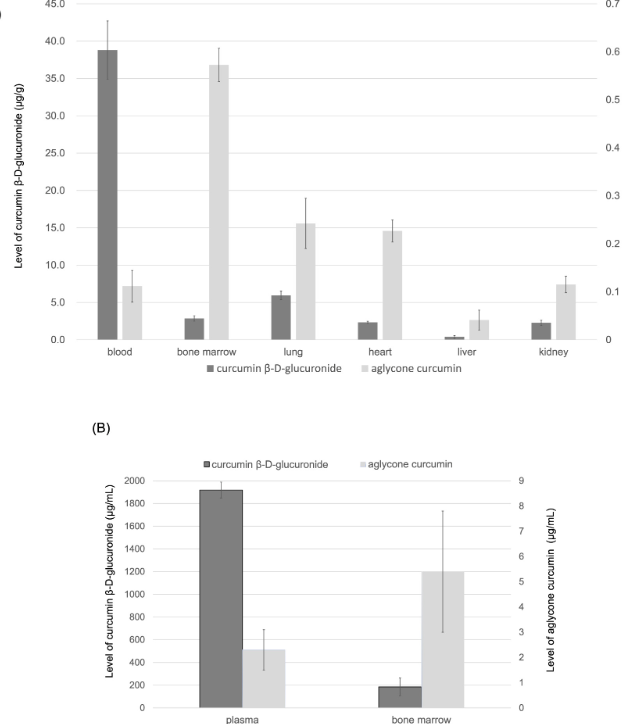
Curcumin levels in blood and major organs of mice and cynomolgus monkeys after intravenous injection of TBP1901
Because TPB1901 can be converted into higher curcumin levels in the bone marrow, the researchers believe that multiple myeloma may be a target disease that TPB1901 can treat. So by intraperitoneally injecting TBP1901 (30 or 90mg/kg, injected 3 times a week for 3 weeks) to multiple myeloma model mice, to evaluate the curative effect of TBP1901, and simultaneously injecting bortezomib (multiple myeloma) intraperitoneally to the model mice Therapeutic drug for myeloma) (1 mg/kg, injected twice a week for 3 weeks) served as a control.
The results showed that after 21 days, the tumor area of the myeloma model mice injected with TBP1901 shrank from 70mm3 to 61.3mm3 (30mg/kg TBP1901), 44.8mm3 (90mg/kg TBP1901). The tumor area of the mice in the bortezomib group shrank to 97.4mm3. This shows that TBP1901 can significantly reduce the tumor volume of multiple myeloma model mice, and the effect is better than bortezomib.
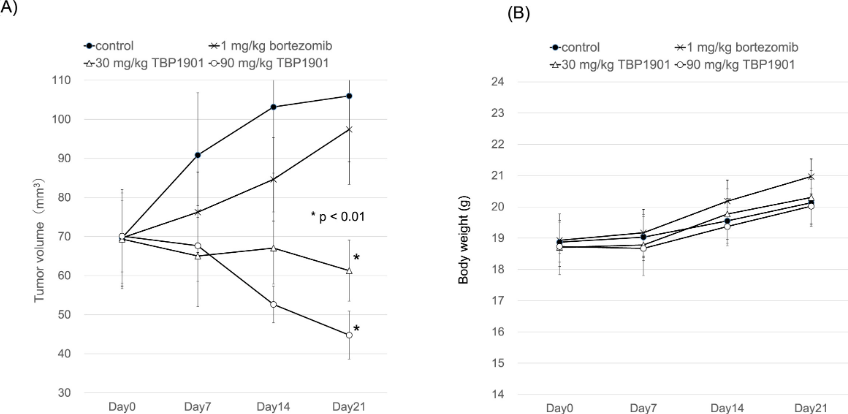
Efficacy of TBP1901 on multiple myeloma model mice
The researchers also found that TBP1901 had little inhibitory effect on multiple myeloma cells in vitro, but curcumin inhibited their proliferation. This indicates that the antitumor effect of TBP1901, a prodrug of curcumin, does not come from itself, but is caused by conversion into curcumin.
In summary, this study found that TBP1901, as a prodrug of curcumin, had significant antitumor effects, and found that GUSB played a key role in the conversion of TBP1901 to curcumin. "In order to take advantage of the high conversion rate of TBP1901 in bone marrow, it is necessary to apply it to the clinical application of bone marrow diseases such as multiple myeloma," the researchers said.
references:
1. Abe, Tomoyuki et al. “Pharmacologic characterization of TBP1901, a prodrug form of aglycone curcumin, and CRISPR-Cas9 screen for therapeutic targets of aglycone curcumin.” European journal of pharmacology vol. 935 (2022): 175321. doi:10.1016/j.ejphar.2022.175321.
